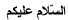I'm still not sure what the triangles are though...

laying:
The triangle is just a way of expressing the equations you have written in another form.
this makes it easier to see the equation and the -/+ values, and which way is negative and which is positive.
CH4(g)+2O2(g) --> CO2(g) + 2H2O(l, just to make it easier)
the enthalpy of a recation is defined as
the enthalpy of the products minus the enthalpy of the reactants(reagents)
1 mol × (-393 kJ/mol) + 2 mol × (-286 kJ/mol)
minus
1 mol × ( -74,8 kJ/mol) + 2 mol × 0 (because the enthalpy of an elemnet (oxygen) equals zero)
equals
-890 kJ
890kJ is the amount of energy released from burning 1 mole of methane. As you know burning produces heat - releases energy.
CH4 + 2O2
CO2 2H2O
Imagine arrows to the (CO2) and (2H2O) coming from (CH4 + 202) - 2 separate ones: 1 to CO2 and one to 2H2O.
AND ALSO from (CO2) to (2H2O) - I cant seem to get them here.
The enthalpy change that you need to work out can be clearly seen.
Insert the temperature values given for each reaction, and work out the one you need to find.
If you need to go in the oppostie direction, then you must use the OPPOSITE temperature sign. So if its positive then you use negative...and the other way around.
Also remember to use the equation.
Delta H is a change in enthalpy - energy. Delta T is a change in temperature
.
OOPs sorry, i got it the other way around....mustve been tired that day, thanks for the correction 'whatsthe point'.
if u dont get it, PM me the question...ill hav a crack at it for you....i was jus tellin the theory behind it.